Materials & Chemicals

Pure, Cyclically-Constrained Gamma-Amino Acids for Medicine and Materials
WARF: P100030US02
Inventors: Samuel Gellman, Li Guo, Michael Giuliano
The Wisconsin Alumni Research Foundation (WARF) is seeking commercial partners interested in developing a novel reaction scheme for the production of high yields of stereochemically pure, cyclically-constrained γ-amino acids.
Overview
Unnatural polymers that contain β- and γ-amino acids are known as "foldamers." These foldamers form long-lasting, predictable structures that are very stable and resistant to proteolytic degradation. They can be designed to adopt specific structures that mimic natural products and can be used as improved substitutes for a variety of products based on peptides or proteins in applications such as medicine, materials or general healthcare.
UW–Madison researchers previously developed a highly efficient, three-step method for synthesizing γ2-amino acids. This method enabled the production of high yields of γ-amino acids. However, only a few types of cyclically constrained γ-amino acid residues currently can be incorporated into peptidic oligomers.
UW–Madison researchers previously developed a highly efficient, three-step method for synthesizing γ2-amino acids. This method enabled the production of high yields of γ-amino acids. However, only a few types of cyclically constrained γ-amino acid residues currently can be incorporated into peptidic oligomers.
The Invention
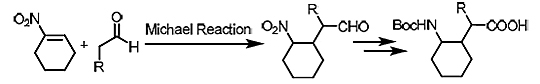
UW–Madison researchers have now developed novel methods for producing cyclically-constrained γ-amino acids that are chiral and highly stereoselective. The researchers also have developed new compositions of cyclically-constrained γ-amino acid residues and reaction intermediates. The methods rely on a reaction that involves the Michael addition of an aldehyde to a cyclic nitroalkene. Oligomers containing the resulting γ-amino acid residues adopt specific conformations and can be used as scaffolds for the construction of biomedically active molecules. These methods provide access to new types of γ-amino acids.
Applications
- Production of stereochemically pure, cyclically-constrained γ-amino acids in high yield
- Medical applications such as antibiotics, hormone mimetics or fat absorption inhibitors
- Material applications including information storage and catalysis
- General healthcare applications like beauty products
Key Benefits
- Provides access to many new constrained γ-amino acid residues which can be incorporated into peptidic oligomers
- Facilitates the production of non-natural amino acids and peptidic oligomers with specific conformations that are useful for applications in medicine, materials and general healthcare
Additional Information
For More Information About the Inventors
Related Technologies
Related Intellectual Property
Publications
- Guo L., Chi Y., Almeida A.M., Guzei I.A., Parker B.K. & Gellman S.H. 2009. Stereospecific Synthesis of Conformationally Constrained Gamma-Amino Acids: New Foldamer Building Blocks That Support Helical Secondary Structure. J. Am. Chem. Soc. 131, 16018-20.
Tech Fields
For current licensing status, please contact Rafael Diaz at [javascript protected email address] or 608-960-9847
Figures