Drug Discovery & Development

Improved Asymmetric Hydroformylation of Therapeutics Using Novel Bisphosphines
WARF: P04447US
Inventors: Clark Landis, Thomas Clark, Jerzy Klosin
The Wisconsin Alumni Research Foundation (WARF) is seeking commercial partners interested in developing a new class of bisphosphines for use in hydroformylation.
Overview
Asymmetric synthesis is important in the pharmaceutical industry because frequently only one optically active isomer, or enantiomer, is therapeutically active. Therefore, it is often desirable to selectively produce one particular enantiomer over its mirror image. One example of such a pharmaceutical product is the anti-inflammatory drug Naproxen. While the (S)-enantiomer is a potent anti-arthritic agent, the (R)-enantiomer is a liver toxin.
Special procedures must be followed to produce the desired enantiomer rather than optically inactive racemic mixtures, in which equal amounts of each mirror image enantiomer cancel each other out. To obtain the desired enantiomer from such a racemic mixture, the mixture must be separated using laborious and expensive processes that result in low yields of the desired enantiomer.
More cost-effective is the synthesis of the desired enantiomer in a catalytic, enantioselective transformation. Catalytic hydroformylation of alkenes is used widely for commodity-scale production of achiral or racemic aldehydes. In principle, catalysts that are chiral by virtue of attached chiral ligands could enable cost-effective synthesis of chiral aldehydes from alkenes by asymmetric hydroformylation. However, existing chiral ligands produce chiral catalysts that are limited in scope and predictability of performance. A wider range of chiral ligands with improved activity and selectivity is needed.
Special procedures must be followed to produce the desired enantiomer rather than optically inactive racemic mixtures, in which equal amounts of each mirror image enantiomer cancel each other out. To obtain the desired enantiomer from such a racemic mixture, the mixture must be separated using laborious and expensive processes that result in low yields of the desired enantiomer.
More cost-effective is the synthesis of the desired enantiomer in a catalytic, enantioselective transformation. Catalytic hydroformylation of alkenes is used widely for commodity-scale production of achiral or racemic aldehydes. In principle, catalysts that are chiral by virtue of attached chiral ligands could enable cost-effective synthesis of chiral aldehydes from alkenes by asymmetric hydroformylation. However, existing chiral ligands produce chiral catalysts that are limited in scope and predictability of performance. A wider range of chiral ligands with improved activity and selectivity is needed.
The Invention
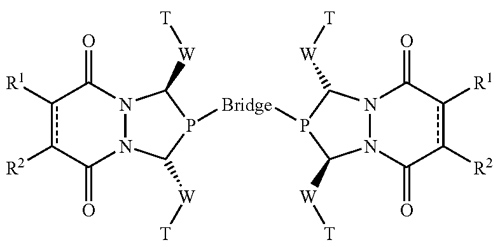
UW–Madison researchers have developed a method for using a new class of bisphosphines as ligands in catalytic transformations of alkenes. The method involves subjecting an alkene to an asymmetric reaction such as hydroformylation, which is carried out in the presence of a bisphosphine ligand catalyst. The bisphosphine ligand is synthesized according to the formula depicted in the figure, or as the opposite enantiomer of the formula. Using this process, asymmetric hydroformylation reactions resulting in 90 percent or greater of the desired enantiomer are possible. The ligand structure has a modular design, which can be varied systematically to obtain the best results for any given alkene substrate, and can be synthesized efficiently from readily available raw materials.
Applications
- Drug production
- Hydroformylation reactions for selective production of optically active aldehydes
- Catalysts with high activity and regioselectivity
Key Benefits
- Allows hydroformylation reactions to be industrially useful due to high catalyst activities and enantioselectivities
- Provides flexible structural design that can be customized for any alkene substrate
- Uses readily available raw materials for ligand synthesis
Additional Information
For More Information About the Inventors
Related Technologies
Publications
- Clark T.P., Landis C.R., Freed S.L, Klosin J. and Abboud K.A. 2005. Highly Active, Regioselective, and Enantioselective Hydroformylation with Rh Catalysts Ligated by Bis-3,4-diazaphospholanes. J. Am. Chem. Soc. 127, 5040-5042.
Tech Fields
For current licensing status, please contact Jennifer Gottwald at [javascript protected email address] or 608-960-9854
Figures