Leveraging radiopharmaceuticals against the most prevalent form of the disease
Overview
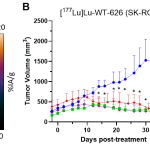
Researchers at the UW-Madison School of Medicine and Public Health and WARF Therapeutics have developed radiopharmaceuticals for the detection and treatment of clear cell renal cell carcinoma (ccRCC). In preclinical studies, the drug demonstrated unprecedented tumor uptake and on-target residence time at seven days along with rapid blood clearance, demonstrating the potential for a best-in-class profile. Researchers are currently conducting proof-of-concept efficacy studies in an animal model of ccRCC (SK-RC-52 xenograft). Interim data can be found below with final survival results expected in spring 2024.
Problem
More than 62,000 Americans and 250,000 people worldwide are diagnosed with ccRCC – the most prevalent form of kidney cancer – every year. While treatment options exist, those for later stages of the disease are not very effective. In fact, current treatments for stage 4 ccRCC have a five-year relative survival rate of only 18 percent.
Solution
The UW-Madison and WARF Therapeutics team developed a radiopharmaceutical that targets the carbonic anhydrase 9 (CA9) protein and can be used for therapeutic and diagnostic applications. More than 95 percent of ccRCC patients have overexpression of this protein at the tumor site.
The researchers anticipate that the radiopharmaceutical could detect and treat related metastases, the main cause of death in cancer. The drug may have additional applications in other CA9-presenting cancers including non-small cell lung, pancreatic, bladder, breast, ovarian, prostate and head and neck cancers.
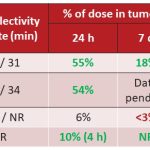
WARF Therapeutics candidates have best-in-class potential
Opportunity
The estimated total market for RCC treatments in 2028 is $12.5B worldwide and $7B U.S.
Traction
Researchers have validated the radiopharmaceutical in preclinical in vitro and in vivo imaging studies. They expect results from animal efficacy studies in spring 2024.
Principal Investigator
 Learn more about Reinier Hernandez, Assistant Professor of Medical Physics. Hernandez harnesses the power of radionuclides to tackle problems in diverse areas of biomedicine. His lab focuses on developing and translating novel radiopharmaceuticals to diagnose and treat cancer and other debilitating diseases.
Learn more about Reinier Hernandez, Assistant Professor of Medical Physics. Hernandez harnesses the power of radionuclides to tackle problems in diverse areas of biomedicine. His lab focuses on developing and translating novel radiopharmaceuticals to diagnose and treat cancer and other debilitating diseases.
Applications
- Radiopharmaceutical for the detection and treatment of clear cell renal cell carcinoma
- Additional indications include non-small cell lung, pancreatic, bladder, breast, ovarian, prostate and head and neck cancers.
Key Benefits
- Both detects and destroys ccRCC tumors and metastases
- Potential to be best in class
- Addresses high unmet need for effective treatment of metastatic ccRCC
- Targets radiotherapy directly to tumor site
- Fewer side effects than traditional radiotherapy
- Potential application in seven additional cancers
Presentations
UW-Madison Partners