Clean Technology

Producing Olefins for Use in Gasoline, Jet and Diesel Fuels from Chemicals Obtained from Levulinic Acid
WARF: P100099US01
Inventors: James Dumesic, David Martin Alonso, Jesse Bond, Dong Wang, Ryan West
The Wisconsin Alumni Research Foundation (WARF) is seeking commercial partners interested in developing a process and apparatus to produce hydrocarbons from aqueous solutions of lactones, hydroxyl-carboxylic acids, alkene-carboxylic acids or alcohols.
Overview
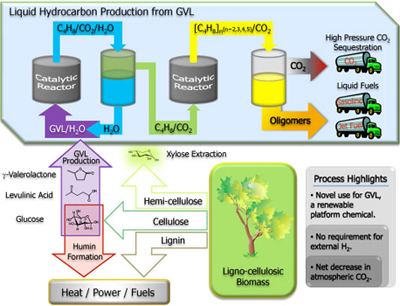
Levulinic acid is a biomass-derived compound that can be obtained inexpensively in high yields from waste cellulose-containing materials. It has been identified as a top biomass-derived chemical due to its ease of production for both five and six carbon sugars and its useful functional groups, a ketone and a carboxylic acid. Levulinic acid can be used to form other, more valuable chemicals including gamma-valerolactone (GVL).
GVL is valuable as a renewable platform molecule with potential in developing both renewable energy and chemicals. GVL retains high energy content and performs comparably to ethanol as a fuel blending agent. However, characteristics such as high water solubility, blending limits for use in conventional combustion engines and lower energy density compared to petroleum-derived fuels have limited the use of GVL in the transportation sector. Therefore, a method is needed to convert lactones such as GVL into liquid alkenes or alkanes with molecular weights targeted for direct use in transportation.
GVL is valuable as a renewable platform molecule with potential in developing both renewable energy and chemicals. GVL retains high energy content and performs comparably to ethanol as a fuel blending agent. However, characteristics such as high water solubility, blending limits for use in conventional combustion engines and lower energy density compared to petroleum-derived fuels have limited the use of GVL in the transportation sector. Therefore, a method is needed to convert lactones such as GVL into liquid alkenes or alkanes with molecular weights targeted for direct use in transportation.
The Invention
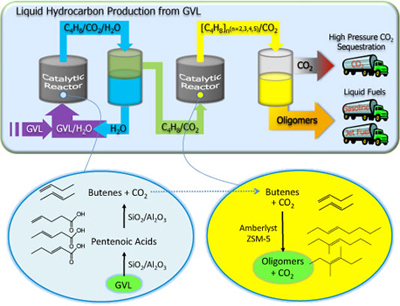
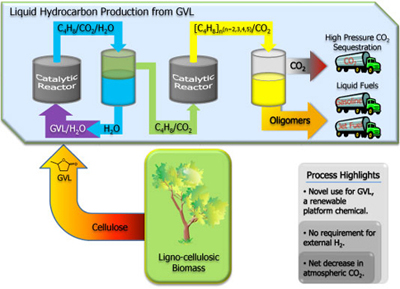
UW-Madison researchers have developed a method and apparatus for producing olefins (unsaturated hydrocarbons) in the C8 to C16 range from GVL. The method involves two tubular flow reactors and an inter-stage separator in a single catalytic system. The chemical transformation proceeds via conversion of GVL to an n-butene, which is then introduced into a second reactor where the butene is converted via acid catalyzed oligomerization to higher molecular weight olefins (C8 and longer). High pressure CO2 is an additional by-product of the reaction.
In addition to GVL, lactones, hydroxyl-carboxylic acids, alkene-carboxylic acids, alcohols or a mixture thereof can be reacted using this method to produce longer-chain olefins. The olefins produced with this method are of carbon chain-length and molecular weight suited for use in gasoline, jet and diesel fuels.
In addition to GVL, lactones, hydroxyl-carboxylic acids, alkene-carboxylic acids, alcohols or a mixture thereof can be reacted using this method to produce longer-chain olefins. The olefins produced with this method are of carbon chain-length and molecular weight suited for use in gasoline, jet and diesel fuels.
Applications
- Olefins in suitable molecular weight range can be used in transportation fuels.
- High pressure CO2 stream can be sequestered or used in further processing.
Key Benefits
- Produces olefins from GVL obtained from biomass, an inexpensive and renewable resource
- Converts GVL to olefins in an integrated system requiring no additional purification
- Synthesizes long-chain olefins (C8+) at high yields and selectivities
Additional Information
Related Technologies
Publications
For current licensing status, please contact Jennifer Gottwald at [javascript protected email address] or 608-960-9854
- Kunkes E.L., Simonetti D.A., West R.M., Serrano-Ruiz J.C., Gärtner C.A. and Dumesic J.A. 2008. Catalytic Conversion of Biomass to Monofunctional Hydrocarbons and Targeted Liquid-Fuel Classes. Science 322, 417-421.
- Bond J.Q., Martin-Alonso D., Wang D., West R.M. and Dumesic J.A. 2010. Integrated Catalytic Conversion of Gamma-Valerolactone to Liquid Alkenes for Transportation Fuels. Science 327, 1110-1114.
- Serrano-Ruiz J.C., Wang D. and Dumesic J.A. 2010. Catalytic Upgrading of Levulinic Acid to 5-Nonanone. Green Chem. 12, 574-577.
Figures