Drug Delivery

Polyplex Delivery System For Proteins, Nucleic Acids And Protein/Nucleic Acid Complexes
WARF: P180158US02
Inventors: Shaoqin Gong, Yuyuan Wang, Krishanu Saha, Amr Abdeen
The Wisconsin Alumni Research Foundation (WARF) is seeking commercial partners interested in developing improved methods for cellular delivery of large biomolecular complexes. The new biocompatible polyplex can deliver larger biomolecular payloads (e.g., S1mplex, RNP with a single-stranded oligonucleotide DNA donor template).
Overview
With an increasing interest in using the CRISPR/Cas9 system for gene editing, there comes a strong need to develop tools for delivering the CRISPR machinery safely and efficiently into cells. Existing non-viral strategies may suffer from one or more disadvantages including high cytotoxicity, poor in vivo stability, large particle size, lack of cell/tissue targeting delivery and variable loading of gene editing machinery. These hurdles continue to limit the ability to fully explore new clinical applications for CRISPR/Cas9 gene editing, especially in living organisms.
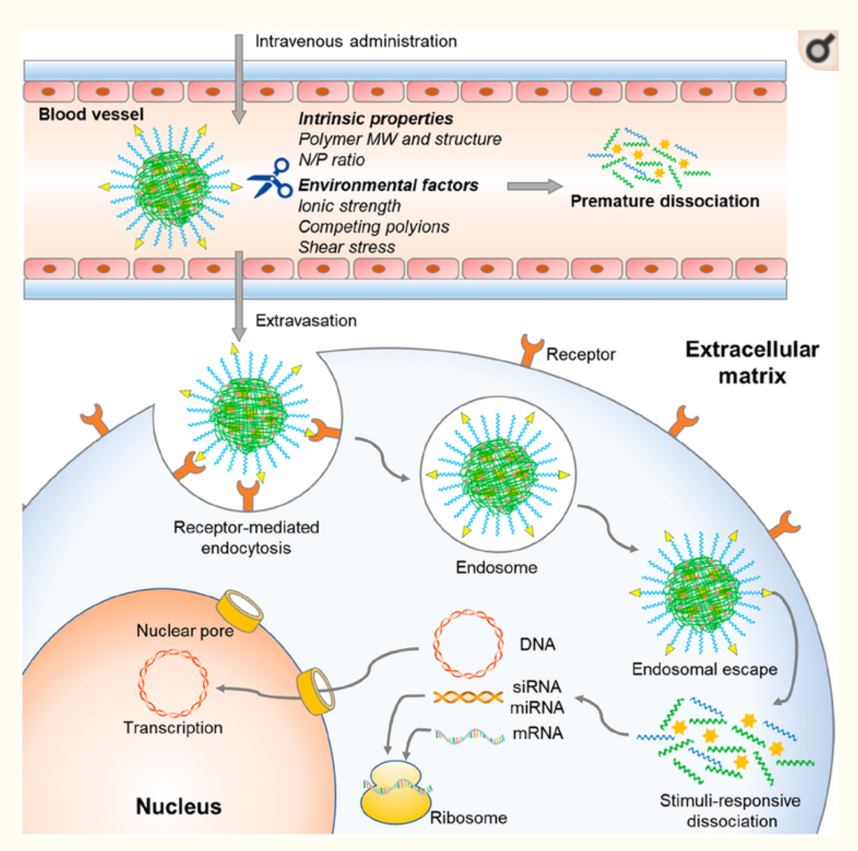
The Invention
UW-Madison researchers have developed an improved biocompatible polyplex that can successfully deliver various biomolecular payloads (e.g., nucleic acids, RNP, RNP together with a single-stranded oligonucleotide DNA donor template). This polyplex contains two polymeric chains that can interact with negatively charged nucleic acid and/or protein payloads – a cationic polymer (poly-β-amino acid, PBAA) containing glutathione (GSH)-responsive disulfide bonds in the backbone and a second polymer that includes modifiable ends (polyethylene glycol, PEG) . Randomly incorporated imidazole groups in the cationic polymer enhance endosomal escape of the polyplex. To further enhance the stability of the polyplexes, adamantane (AD) and β-cyclodextrin (β-CD) are conjugated to the polymers. The crosslinked polyplexes formed by host–guest interaction between β-CD and AD are more stable than non-crosslinked polyplexes in physiological conditions. Once in the cytosol, the disulfide bonds are cleaved by GSH, thereby releasing the protein/nucleic acid from the polyplex. Importantly, at the PEG terminal ends, simple chemistry can be used to add various cellular or tissue-specific targeting ligands and imaging probes.
When compared to a commercially available transfection reagent (Lipofectamine 2000), the polyplex significantly decreased toxicity and showed equal or better nucleic acid transfection efficiency in HEK 293 and RAW 264.7 cells. Testing both sgRNA/Cas9 complex (i.e., RNP) and RNP together with single-stranded DNA complex, the inventors showed that using the polyplex delivery system yielded superior gene knockout and gene editing results.
When compared to a commercially available transfection reagent (Lipofectamine 2000), the polyplex significantly decreased toxicity and showed equal or better nucleic acid transfection efficiency in HEK 293 and RAW 264.7 cells. Testing both sgRNA/Cas9 complex (i.e., RNP) and RNP together with single-stranded DNA complex, the inventors showed that using the polyplex delivery system yielded superior gene knockout and gene editing results.
Applications
- Delivery of large biomolecular payloads into cells (e.g., DNA, mRNA, sgRNA/Cas9 RNP, and RNP together with ssODN)
Key Benefits
- Versatile nanodelivery system capable of safely and efficiently delivering a wide range of biomolecules (e.g., DNA, mRNA, CRISPR RNP, RNP together with a DNA repair template)
- Significantly reduced toxicity compared to existing transfection reagents
- Tunable platform for application-specific modifications (via PEG moieties)
Additional Information
For More Information About the Inventors
Publications
- Wang et al. Versatile Redox-Responsive Polyplexes for the Delivery of Plasmid DNA, Messenger RNA, and Crispr-Cas9 Genome-Editing Machinery. 2018. ACS Applied Material Interfaces 10(38), 31915-31927.
- Wang et al. Enhancing the In Vitro and In Vivo Stabilities of Polymeric Nucleic Acid Delivery Nanosytems. 2019. Bioconjugate Chemistry 30(2), 325-337.
Tech Fields
For current licensing status, please contact Jennifer Gottwald at [javascript protected email address] or 608-960-9854
Figures