Light/Expansion Microscopy
Overcome the diffraction limit inherent in light microscopy. Learn how new breakthroughs in expansion microscopy (ExM) enable investigators to examine small structures previously beyond the grasp of light microscopy.
Optimized Economical and Modulatable Isotropic Expansion Microscopy
Aussie Suzuki, Mark Burkard, Roshan Norman, Emma Recchia
This optimized set of protocols for better expansion of expansion microscopy (ExM) samples allows for 4 to 10-fold expansion of tissue cells and human organoid without expensive equipment or materials.
Light/Fluorescence Microscopy
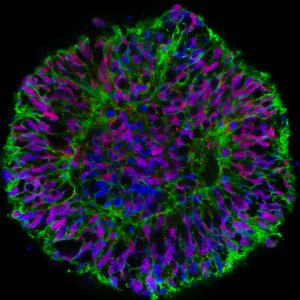
Fluorescent microscopes require frequent calibration, testing, and maintenance. Our researchers have developed novel tools that significantly simplify this process to ensure maximum performance from your instruments.
Microtubule Labeled Slides Used for Calibration of Various Fluorescence Microscopes
Aussie Suzuki
The microtubule slide can be used to calibrate any optical microscope, including light, confocal and super-resolution instruments.
Cryo-Electron Microscopy (cryo-EM)
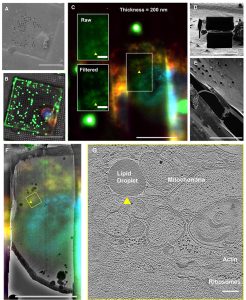
Sample preparation traditionally has been one of the most difficult and limiting aspects of cryo-electron microscopy (cryo-EM). We have developed new preparation methods that simplify this process while retaining sample integrity.
Biomolecular Vapor Deposition (BVD) and Grid Holder/Mounting System for In Vacuo Preparation of Cryo-Electron Microscopy Samples
Joshua Coon, Michael Westphall
This new cryo-electron microscopy (cryo-EM) sample preparation method combines biomolecular vapor deposition (BVD) with an improved TEM grid handling system.
Freezing and Jacketing Gas-Phase Biomolecules with Amorphous Ice for Electron Microscopy
Joshua Coon, Michael Westphall
The new cryo-electron microscopy (cryo-EM) sample preparation method, called biomolecular vapor deposition (BVD), is a gas-phase sample preparation system that uses mass spectrometry to mitigate many of the shortfalls associated with cryogenically fixing biological samples in amorphous ice.
Gas Phase Sample Preparation For Cryo-Electron Microscopy
Joshua Coon, Michael Westphall
This new cryo-electron microscopy (cryo-EM) sample preparation method addresses the limitations of current preparation methods and uses mass spectrometry to purify proteins and protein complexes.
Single-Molecule Microscopy
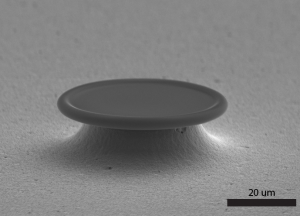
Expand the use of single molecule microscopy beyond fluorescent molecules. These technologies enable microscopy to be performed on non-fluorescent single molecules, allowing study on a broader range of molecules.
All-Glass Optical Microresonator for Single Molecule Spectroscopy and More
Randall Goldsmith, Kassandra Knapper, Kevin Heylman
The improved optical microresonator platform shows superior biocompatibility and performance.
Microcavity Method for Single Molecule Spectroscopy
Randall Goldsmith, Kevin Heylman
The new microcavity-based method enables detection, identification and real-time analysis of single molecules and particles.
Applied Microscopy
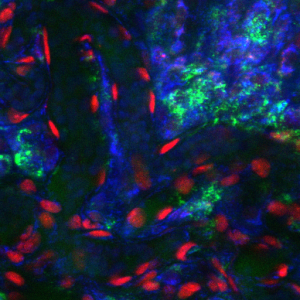
T cell activation plays a critical role in immunotherapy. Learn how new tools in the sorting and classification of T cells enable medical professionals to better characterize T cells and improve therapeutic efficacy.
Algorithms to Classify T Cell Activation by Autofluorescence Imaging
Melissa Skala, Anthony Gitter, Zijie Wang, Alexandra Walsh
The method and algorithms to non-invasively detect T cell activation by imaging NAD(P)H can be applied to NAD(P)H images taken with commercial imaging flow cytometers/sorters and fluorescence microscopes.
Method and Device to Screen and Sort Cancer Immunotherapy Cells
Melissa Skala, Alexandra Walsh
This non-invasive method uses the autofluorescence signals of NAD(P)H and FAD to determine T cell activation state without the use of contrast agents or requiring tissue/cell fixation.
Microscopy cores at UW-Madison
Cryo-Electron Microscopy Research Center (CEMRC)
The CEMRC provides instrumentation, technical assistance, training and access to cryo-EM for the UW-Madison research community. The CEMRC manages and operates four cryo-microscopes for data collection by single particle, tomography and micro-ED. The microscopes are overseen by experienced staff who offer consultation and training in negative-stain and vitrified sample preparation, single particle analysis, tomography, data processing and additional computational support.
Translational Research Initiatives in Pathology (TRIP) Lab
TRIP provides high quality services in cellular, molecular, quantitative and computational pathology to the UW campus research community and beyond. TRIP offers histological, histochemical, molecular and imaging analyses services such as tissue microarray (TMA) creation, imaging of slides in RGB and multispectral formats, IHC/ISH and cell line authentication on a fee for service basis.
Newcomb Imaging Center (NIC)
NIC is a space for research and education in the department of botany that offers expertise, instruction and access to instrumentation in modern microscopy to the community of scientists at UW-Madison.
Optical Imaging Core
The UW-Madison Optical Imaging Core offers investigators and biotech partners training and accessibility to advanced imaging technology.
UW Vision Research Core
The UW-Madison Vision Research Core is funded by the National Eye Institute (NEI) and provides vision researchers three distinct service areas: quantitative molecular biology, pathology & imaging and animal models & eye organ culture.
Nanoscale Imaging and Analysis Center (NIAC)
NIAC provides state-of-the-art instrumentation, support facilities and expert technical assistance for microscopy and microanalysis to support research and education in materials.
Scanning Election Microscope (SEM) Lab
Scanning Electron Microscopy is an excellent method for providing high spatial resolution images on a wide range of samples. SEM provides high depth of focus images of 3-dimensional structures as well as compositional contrast images.